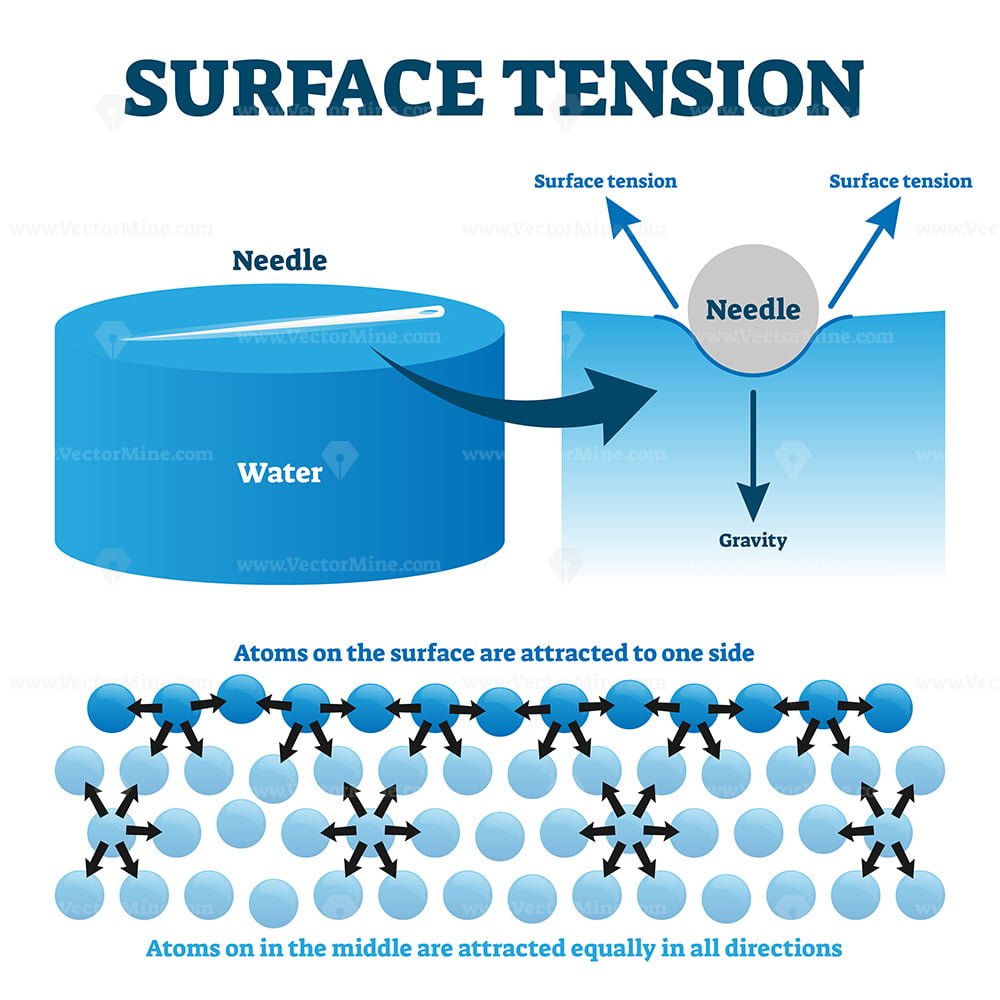
What Does Surface Tension Form . surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. discover how cohesion between water molecules forms surface tension,. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are. surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force.
from vectormine.com
surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force. discover how cohesion between water molecules forms surface tension,. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. The molecules on the surface of a liquid are. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces.
Surface tension explanation vector illustration diagram VectorMine
What Does Surface Tension Form The molecules on the surface of a liquid are. surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force. discover how cohesion between water molecules forms surface tension,. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The molecules on the surface of a liquid are. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane.
From thechemistrynotes.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences What Does Surface Tension Form surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The molecules on the surface of a liquid are. surface tension (denoted with the. What Does Surface Tension Form.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free What Does Surface Tension Form discover how cohesion between water molecules forms surface tension,. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension (denoted with the greek variable gamma). What Does Surface Tension Form.
From sophisticatedthoughtscom.wordpress.com
The Science Behind Bubbles (1/3) Sophisticated Thoughts What Does Surface Tension Form surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. discover how cohesion between water molecules forms surface tension,. surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force. surface. What Does Surface Tension Form.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free What Does Surface Tension Form surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon that occurs due to the cohesive. What Does Surface Tension Form.
From www.vrogue.co
Surface Tension Definition Examples Causes And Measur vrogue.co What Does Surface Tension Form surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension,. What Does Surface Tension Form.
From www.slideserve.com
PPT Chapter 5 Liquids and Solids PowerPoint Presentation, free What Does Surface Tension Form surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are. surface tension is the energy, or work,. What Does Surface Tension Form.
From www.youtube.com
What are the application of surface tension? YouTube What Does Surface Tension Form surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along. What Does Surface Tension Form.
From www.youtube.com
How does Surface Tension work? YouTube What Does Surface Tension Form surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular. What Does Surface Tension Form.
From civilchapola.com
What's Surface Tension in Fluid Mechanics Water Droplet & Soap Bubble What Does Surface Tension Form surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique. What Does Surface Tension Form.
From www.edumple.com
Surface tension, Viscosity Notes for JEE Chemistry EduMple What Does Surface Tension Form surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float. What Does Surface Tension Form.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Does Surface Tension Form The molecules on the surface of a liquid are. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension (denoted with the greek variable gamma) is defined as the ratio of the surface force f to the length d along which the force.. What Does Surface Tension Form.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Does Surface Tension Form surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float. What Does Surface Tension Form.
From delprofedefisica.blogspot.com
Física Tensión superficial What Does Surface Tension Form surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. The molecules on. What Does Surface Tension Form.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Does Surface Tension Form surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float. What Does Surface Tension Form.
From www.sexiezpix.com
Ppt Motivation For Studying Fluid Mechanics Powerpoint SexiezPix Porn What Does Surface Tension Form surface tension, the amount of energy required to increase the surface area of a liquid, is a unique property determined by intermolecular forces. surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules.. What Does Surface Tension Form.
From www.pinterest.com
What is Surface Tension? Surface Tension, Solid Surface, Bid, Behavior What Does Surface Tension Form The molecules on the surface of a liquid are. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension (denoted with the greek variable gamma) is defined as the. What Does Surface Tension Form.
From www.toppr.com
The surface tension is T=dfrac{F}{l}, then the dimensions of surface What Does Surface Tension Form The molecules on the surface of a liquid are. discover how cohesion between water molecules forms surface tension,. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. surface tension is the energy, or work,. What Does Surface Tension Form.
From www.vocationindia.com
Dimension of Surface Tension & its Derivations What Does Surface Tension Form surface tension, property of a liquid surface displayed by its acting as if it were a stretched elastic membrane. surface tension is the elastic tendency of fluid surfaces that lets insects to float on water. surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. discover how cohesion between water molecules. What Does Surface Tension Form.